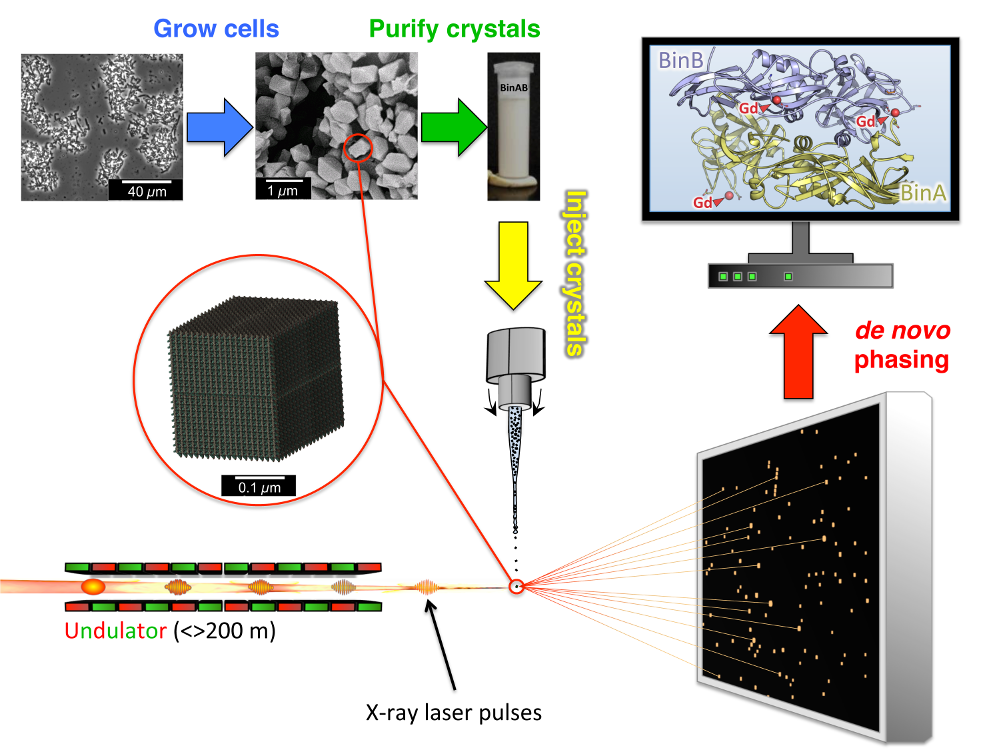
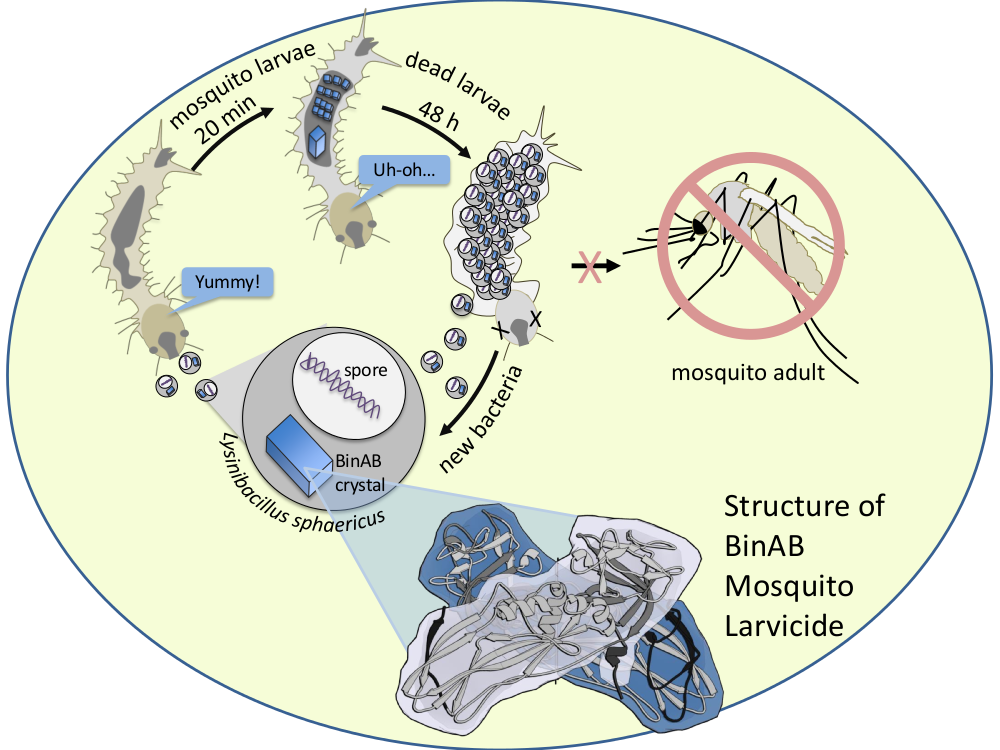
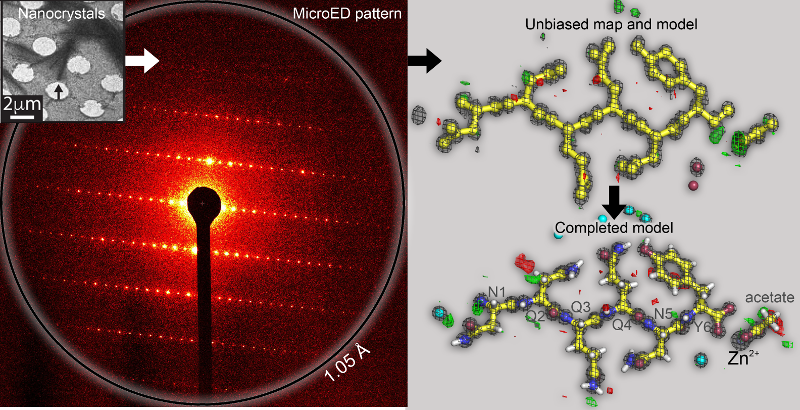
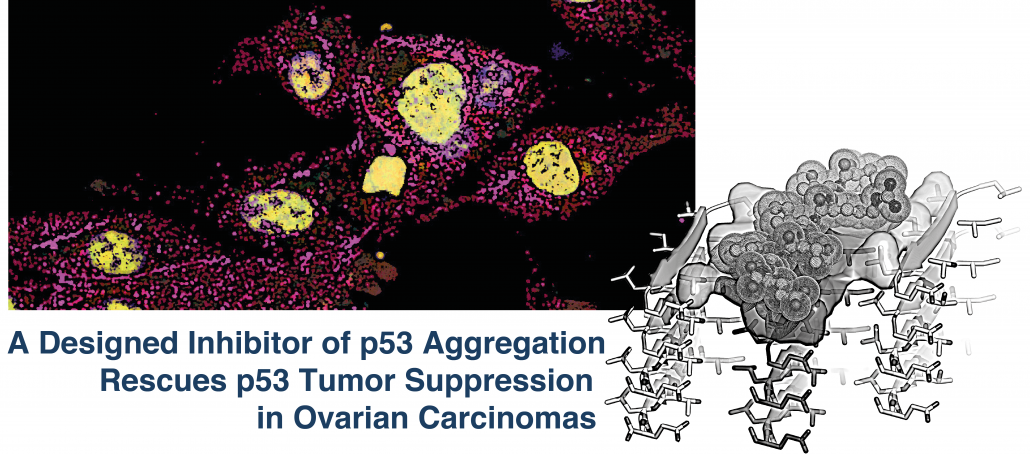
Protocols for some Crystallization Stock Solutions
Dan Anderson
We have our own water machine to make HPLC grade water. Use this very pure water, and chemicals of ACS grade or better.
Ammonium Sulfate, saturated solution: Weigh about 80 grams of enzyme grade ammonium sulfate. In a 250ml beaker, stir the solid into 100ml of wa ter. Heat the mixture to dissolve the last of the solid. The final volume is about 120ml. Get a 0.2um filter wet with water before use, then filter the solution while it is still hot. The concentration equilibrates several days later, when crystals appear.
Sodium Citrate buffers: Citrate buffers become very hot during titration with NaOH. The pH’s of buffers that get hot are not reproducible. To make a 0.5M citrate buffer without heating, first separately prepare 0.5M citric acid, and 0.5M Na3 citrate. Mix them while watching the pH with a pH meter. No matter how you mix them, it’s still 0.5M total citrate. Calibrate the pH meter around the pH of interest. Our pH meter automatically recognizes pH 1.68 standard buffer, in addition to the more familiar standards.
Lithium Citrate buffers: This one is really a pain because LiOH is not very soluble, and it fizzes as though part of it is LiHCO3. If you need a series of Li citrate buffers, make the highest pH one first, to final concentration, then mix that with the same concentration of citric acid to back-titrate to the low er pH values. The volume of the highest pH buffer has to be enough to make all the lower pH buffers.
Filtration of 2M Lithium Sulfate: The 0.2um filter has to be wet first.
Lithium chloride: LiCl is soluble to at least 8M. Dissolving LiCl is exothermic. Preparation of the concentrated solution generates concentrat ed heat that can melt Parafilm and make a warm mess. Add the LiCl to the water slowly. It takes a long time for 8M LiCl to wet a cellulose acetate filter membrane. 5M LiCl filters much faster.
Polyethylene Glycol: Weigh the desired amount, put the solid into a graduated cylinder of the desired volume. Add water to less than the final volume. Seal with Parafilm. Mix on a Nutator until dissolved, then add water to final volume. For example, for a 30% stock solution, weigh 30g, put it into a 100ml graduat ed cylinder with water up to about the 90ml mark, seal and Nutate. Later on, add water to 100ml.
Polyethylene Glycol 1000: PEG1000 is poured into its container as a melt; it is not flaked or powdered like the other PEG’s. To make a PEG1000 solution, warm the PEG1000 container in a water bath to melt it, then pour out the desired amount. Dissolve it in water before it solidifies, otherwise it will take a lon g time to dissolve.
4M Na/K Phosphate: I don’t know if this results in anybody’s “standard” pH. Decide what final volume you want, then weigh enough of NaH2PO4, Na2HPO4, KH2PO4, K2HPO4 to make that final volume 1M of each one. Add a little at a time of each one to water, with stirring. When all 4 are nearly dissolved, add water to reach the final volume.