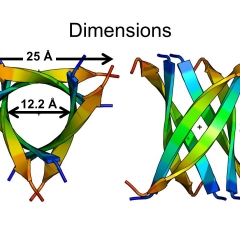
The fee structure is designed to divide the ongoing costs among anticipated users, based on usage of the facility.
This usage covers numerous activities: x-ray data collection, machine maintenance, data collection at home, travel to synchrotrons and data collection there, expert assistance with structure determination (e.g. molecular replacement, heavy atom phasing, refinement), coordinate deposition, structure interpretation and analysis, and figure and manuscript preparation.
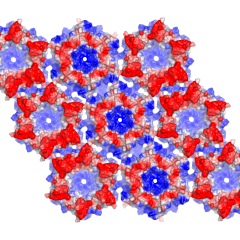
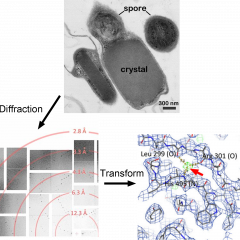
A point system of a unit has been developed to assign usage to participating research groups. INTERNAL X-RAY SERVICE (UCLA) will be charged the approved rate of $105.98 per unit. EXTERNAL X-RAY SERVICE ( NON-UCLA) will be charged $135 per unit. A PI should anticipate a charge of around 20 units per quarter to use every instrument, and materials available in the facility including: FRE+ X-ray generators, HTC image plate detectors, High Power Leica Microscopes with High Resolution CCD Cameras, Korima UV microscope, all Cryogenic Equipment, storage of crystals in Liquid Nitrogen Dewars, storage in the 5 degrees Crystallization Room, data collection at the synchrotron and experienced staff assistance with structure determination.
New users should discuss setting up a recharge account with the XRAY and EM STRUCTURE DETERMINATION Core Director.